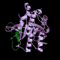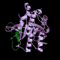|
|
|
PSDB: About Subtilases
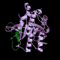
The PSDB provides significant information on gene functions and results of experimental analyses of plant subtilisin-like serine proteases (subtilases).
To get information about plant subtilases use the links listed below.
About Subtilases 
Subtilisin-like proteases (subtilases) are serine proteases characterised by a catalytic triad of three amino acids, namely aspartate, histidine, and serine (Dodson & Wlodawer, 1998). The arrangement of theses catalytic residues is shared with the prototypical subtilisin from Bacillus licheniformis. According to the MEROPS classification, subtilases constitute the S8 family within the SB clan of serine proteases. Subtilases are grouped into two subfamilies, namely those of subtilisins (S8A) and kexins (S8B). Nine subtilases have been characterised in mammals, seven of which belong to the S8B subfamily, the pro-protein convertases (PCs) (Seidah et al., 1999a; Sedan & Chretien, 1999). More recently, two mammalian subtilases were identified within the S8A subfamily. The Site-1-protease (S1P) and the subtilisin-kexin-isozyme-1 (SKI-1) belong to the distinct group of pyrolysins (Siezen and Leunissen, 1997) and have been shown to carry out specific cleavage and processing reactions on sterol regulatory elements, binding proteins and pro-brain-derived neurotrophic factors, respectively (Sakai, 1998; Seidah, 1999a; Seidah, 1999b).
Plant Subtilases: Arabidopsis Subtilases (AtSBT) 
Plant subtilases, together with S1P and SKI-1, belong to the pyrolysin group within the S8A subfamily. The subtilase family in Arabidopsis contains 56 members (AtSBT) and its complexity thus exceeds that of the seven mammalian subtilases by far (Sedan and Chretien, 1999). Despite their prevalence, our current understanding of subtilase function in plants is still very limited. There are evidences for a role in both, general protein turnover as well as highly specific regulation of plant development or responses to environmental challenges.
Functions of Plant Subtilases 
A number of proteases have been purified from plant tissues and classified as subtilases based on their catalytic properties as well as according to their primary structure. Examples include macluralisin (Rudenskaya et al., 1995), taraxalisin (Rudenskaya et al., 1998), plantagolisin (Bogacheva et al., 2001), subtilases from green malt (Terp et al., 2000; Fontanini & Jones, 2002), developing tung fruits (Dyer et al., 1999), bean (Popovic et al., 2002), soybean (Beilinson et al., 2002; Boyd et al., 2002), and Arabidopsis (Hamilton et al., 2003). Most of these enzymes are highly abundant and exhibit broad substrate specificity. Thus a functional involvement in general protein turnover was forecasted for these abundant proteins (Bogacheva, 1999). The primary example for subtilases that function in protein degrading is cucumisin, which constitutes up to 10 % of the soluble protein in melon fruit. It was characterised extensively at the enzymatic level and was also the first subtilase cloned from any plant species (Kaneda & Tominaga, 1975; Yamagata et al., 1994).
The tomato subtilase P69 was initially identified as a pathogenesis-related protein and was shown to be one of the several subtilases that are specifically induced following pathogen infection (Vera & Conejero, 1988; Tornero et al., 1996a; Jordá et al., 1999). P69 processes a leucin-rich repeat cell wall protein in virus-infected tomato plants and thus is one of the very few plant subtilases for which an endogenous substrate has been identified. The direct consequences of this processing event for pathogenesis are still unknown (Tornero et al., 1996b). The P69 enzymes form a distinct subgroup among the 15 subtilases that have hitherto been currently cloned from tomato (Meichtry et al., 1999). Forward genetics has identified subtilases as highly-specific regulators of plant development. In the Arabidopsis SDD1 mutant (stomatal density and distribution 1) the pattern of stomata formation is disrupted resulting in clustering of stomata as well as in a dramatical increase of stomatal density. The gene was identified by positional cloning and found to encode a subtilase (Berger & Altmann, 2000). SDD1 is strongly expressed in meristemoids and guard mother cells, the precursor cells of stomates. The SDD1 protein is probably secreted into the apoplast of the cells where it is thought to act as a processing protease in the generation of signals responsible for stomata density regulation (von Groll et al., 2002). Likewise, the gene disrupted in the ALE1 mutant (abnormal leaf shape 1) was cloned and found to encode a subtilase. ALE1 is required for cuticle formation and epidermal differentiation during embryo development in Arabidopsis. A role for ALE1 was suggested in the generation of peptide signals required for proper differentiation of the epidermis (Tanaka et al., 2001). The mutant phenotypes of SDD1 and ALE1 demonstrate that at least some subtilases carry out highly specific functions in plant development. Their modes of action in the regulation of the respective developmental processes are still unknown but SDD1 and ALE1 may be required for the generation of (poly-)peptide signals which act non-cell autonomously to control plant development.
Although some progress has been made towards understanding the modes of action and functions for several subtilases, this is still unclear for the majority of plant subtilases and includes the model organism Arabidopsis subtilases.
|
|
|
|
|
For suggestions and questions feel free to contact the CSB.DB curator. |
|
|
|
|
|
Requirements
Minimal resolution for optimal view: 1024x768 (without favorites)! Web browser of the sixth generation required, e.g. MS IE 6.0. Javascript must be enabled... more |
|
|